Loading...
Clinical Data Management
Corporate
Online
RESOLVEVING DATA ISSUES
SAS
SAS CDM
SAS CDM Online Training
Training
http://sascdmonlinetraining.blogspot.com/2015/11/the-solution-to-resolve-data-issues.html
As SAS statistical programmers, you can
easily write programs to list all unique values of the gender variable, for example,
to inform the team that an invalid value exists for that variable. Once you can
isolate clinical data issues, they become ‘known’ and can be ‘accounted for’ to
explain differences in expectations and conflicts. Implementing the clinical
data acceptance testing procedure involves developing a collection of single
purpose macros with basic requirements. Once the system is in place for one
clinical study, multiple studies could also be checked as a universal set of
macros since the checks are all repetitive and standard.
The benefits of using these macros are
increased productivity by quickly and easily apply the macros to other clinical
studies, the acceptance of CDM to use the systematic approach method of
communicating common issues/concerns, and the biostatistics department having
more confidence in the raw clinical data. The end result is that deadlines are not
missed since SAS programs do not have to be written defensibly to account for
these data issues.
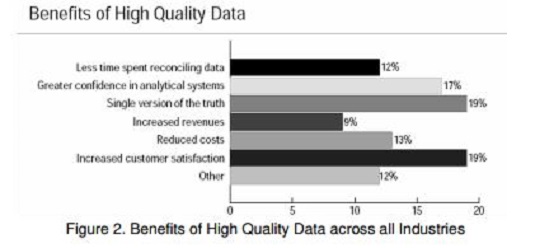
According to the same 2001 survey by the
Data Warehousing Institute in figure 2, the benefits of high quality data across
all industries can be identified below. During the FDA submission process, a
single version of the truth and increased customer satisfaction are very
important to recognize reduced costs and minimum delays to get the drug approved.
These outcomes are well worth the average cost of $20 to $25 per case report
form page or up to 15 % of the clinical research budget to ensure data quality.
Overall, the process flow consists of
accessing raw data, which may contain invalid data, with edit check macros to monitor
data issues so that only valid data is used in the final analysis data sets,
tables, lists and graphs. With this solution, if invalid data is used in the
outcome, then the unexpected results can be explained.
Specifically, the solution involves
these four steps before having the database lock:
1. Specifying Requirements in Data
Management Plan (DMP)
2. Developing and Testing Edit Check
Macros
3. Communicating Results with Clinical
Data Management (CDM)
4. Monitoring the Metrics of Data Issues
Clinical Data Management,
Corporate,
Online,
RESOLVEVING DATA ISSUES,
SAS,
SAS CDM,
SAS CDM Online Training,
Training
Training
7320789590832411441
Post a Comment Default Comments Facebook Comments
Home
item
Blog Archive
Popular Posts
-
The system design is logically separated into three sections a core application section, a section for administration of multiple da...
-
In general, the CDM department may not spend enough resources to check the quality of the data. This is because CDM’s main responsibility ...
-
As SAS statistical programmers, you can easily write programs to list all unique values of the gender variable, for example, to inform the...
-
SAS Clinical Data Management(CDM) is also known as Clinical Data Integration supports the pharmaceutical-industry wants for transformin...
-
Q)What is the therapeutic area you worked earlier? There are so many diff. therapeutic areas a pharmaceutical company can work on and ...